Type “collagen placebo” into Google and you’ll find no shortage of doubt. Some argue that every wrinkle study is funded by the very companies selling the powders. Others point out that collagen is just protein, broken into amino acids like any other steak or soy shake. And many trials, they’ll note, measure outcomes with a “wrinkle score” or elasticity probe that feels softer than hard scientific endpoints.
These concerns are not only valid; they are essential. Without scepticism, the supplement industry drifts into hype. What matters is whether the data, stripped of marketing gloss, still shows that collagen hydrolysates deliver something more than a standard protein shake.
This is not a sales pitch. It is a no-hype audit of the five strongest sceptic arguments—and what the evidence, mechanistic and clinical, really says.
Uncover Advanced Protocols and Launch Access.
For those who champion evidence over hype.
The Building Block Dilemma
First, a recap of why collagen has become synonymous with the beauty and wellness space. Collagen is the most abundant protein in the body, acting as the fundamental scaffold for our skin, bones, muscles, and tendons. However, eating collagen does not directly “put” collagen into your skin. So, what is the logic of supplementation, and where exactly are the sceptics right?
Reason 1 — Funding bias is real
Concede: A fair criticism of collagen research is that many clinical trials showing improvements in skin hydration, elasticity, or wrinkles are industry-funded — and recent independent 2024 meta-analyses [Myung and Park, 2024] confirm that these visible effects are far less consistent when funding bias is removed. This does not mean collagen “does nothing”; it means appearance-level outcomes have been overstated by marketing-driven trial design. Skepticism here is not anti-science — it is precisely what good science demands.
Counter: But dismissing the entire field because of funding is too simple. An independent academic groups, notably in Japan, have published mechanistic work showing that specific collagen-derived peptides, such as Pro-Hyp, appear in blood after ingesting hydrolysed collagen supplements. These peptides stimulate fibroblast activity, the cells reposnsible for collagen production [Iwai et al., 2009; Asai et al., 2020]. These studies don’t rely on self-reported wrinkle scores; they measure plasma peptides, cell signaling, and fibroblast growth.
Action: The skeptical lens should not erase these signals — it should demand higher standards of design: preregistered trials, transparent funding, and objective endpoints.
Reason 2 — “Collagen is just amino acids” (Partly true)
Concede: Sceptics are right that collagen, like any protein, is digested. Much of it does break down into single amino acids—the same building blocks you’d get from meat, soy, or whey.
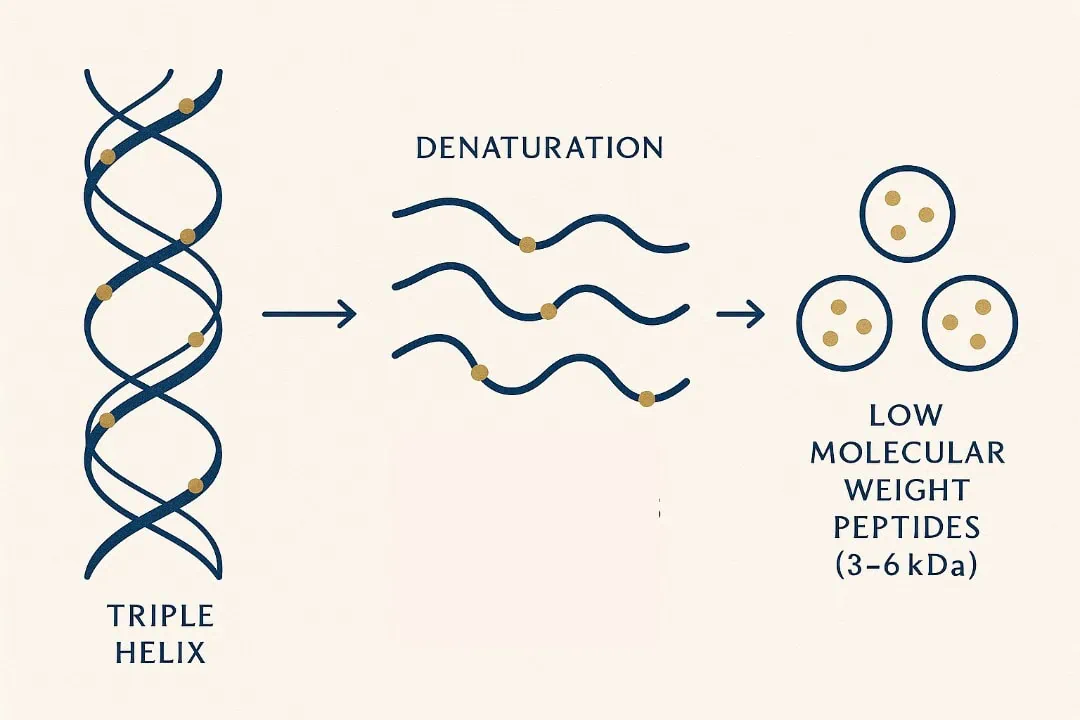
Counter: Collagen possesses a unique biochemical fingerprint: it is exceptionally rich in hydroxyproline. The critical distinction is not just the presence of the amino acid, but the presence of peptides. Among these, Prolyl-Hydroxyproline (Pro-Hyp) is the most studied. It is a dipeptide—a two-amino-acid chain—that your body cannot make from scratch. When collagen is hydrolysed (broken down via enzymes), these specific fragments are liberated. They are absorbed into the bloodstream via the PEPT1 and PEPT2 transporters as intact dipeptides and tripeptides. They don’t just act as “food”; they act as “messengers“
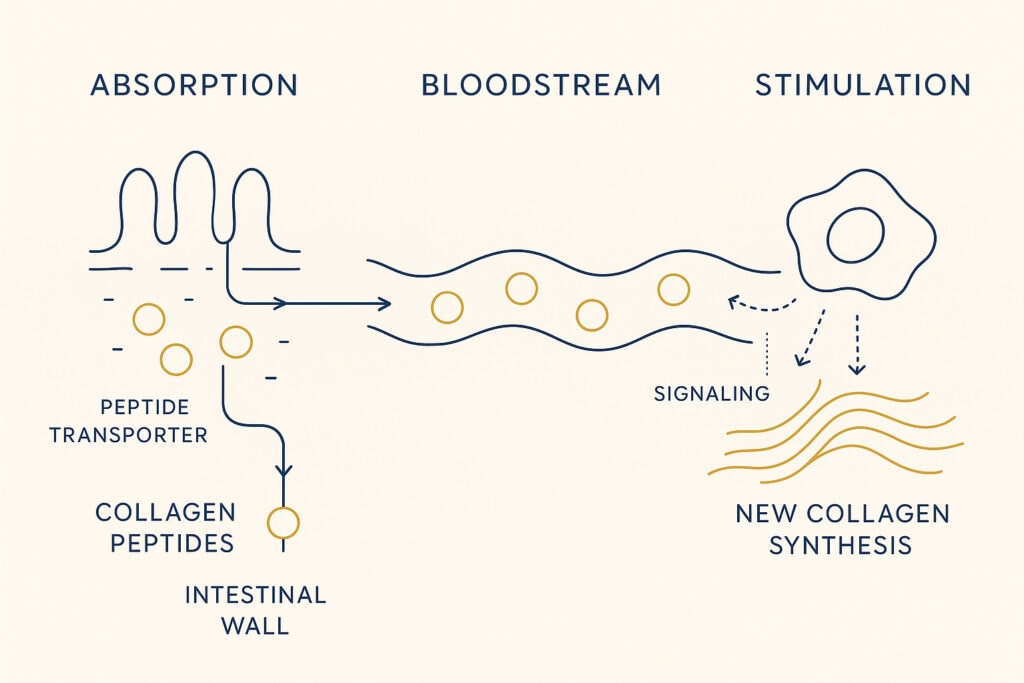
The Signal vs. The Building Block: Think of these peptides as molecular “breadcrumbs” left behind when collagen unravels. Fibroblasts—the cells that build new collagen—read those breadcrumbs as a signal: Time to repair. In lab studies, when fibroblasts are exposed to Pro-Hyp, they become more active, multiplying and moving into the surrounding tissue like builders responding to a repair siren. Researchers have traced this to a molecular switch inside the cell that gets flipped when Pro-Hyp binds — a switch that leads to new collagen being made [Ohara et al., 2010].
Why Molecular Weight Matters: The “size” of the collagen you swallow is the crux of the debate. Most clinical studies use collagen averaging 2–5 kDa. In this range, Pro-Hyp is detected most abundantly in the blood. What matters is not chasing an ultra-specific number, but ensuring the collagen is truly hydrolysed rather than just intact gelatin. Recent research shows that as long as it falls within that 2–5 kDa range, the body yields comparable levels of bioactive peptides. [Nicolina Virgilio et.al, 2024]
Reason 3 — Not all collagen supplements are equal
Sceptics often question the quality of collagen itself. If it comes from animal hides or fish skins, how do you know what else is carried along? Could there be contaminants from the raw material? Could cheap processing leave behind residues? These are not trivial concerns — the source and the manufacturing chain matter as much as the scoop of white powder at the end.
Concede. The category is uneven. Some products are made from poorly documented sources or processed without transparency. Not all collagen supplements are created equal, and consumers should be aware of the differences among collagen products. In those cases, you may be left with bulk protein that dissolves easily but tells you little about its safety or bioactivity.
Counter. What matters is control.
- Marine collagen comes from fish skin or scales. Because fish live in different water environments, there can be variation in trace contaminants like heavy metals or microplastics. The concern isn’t that marine collagen is unsafe, but that quality depends on how carefully the raw material is selected and tested [Cammilleri et al., 2021].
- Bovine collagen is widely used and, in the EU, tightly regulated. But here too, safety relies on documented sourcing — hides processed under food-grade standards, not industrial by-products.
Action. The way to separate commodity powder from credible product is not by marketing promises but by transparency. Brands that identify their suppliers, provide lot-level Certificates of Analysis, and specify molecular weight ranges (≤5 kDa) show they are taking quality seriously. You can read more about (Marine vs Bovine collagen) in our dedicated guide here.
Reason 4 — Outcomes like skin elasticity often sound “soft” (and sometimes are)
Concede. A lot of collagen trials are short, some are brand-funded, and many include self-reported questionnaires about how people feel their skin looks. That opens the door to placebo — if you expect a glow, you might report one. Fair point.
Counter: Good studies use validated instruments (like corneometers) that provide numbers, not just impressions.
In Joint research, a related collagen-rich tissue, scientists track objective markers of collagen turnover:
- CTX-II — a fragment that indicates cartilage collagen is breaking down
- PIIANP — a fragment that shows new cartilage collagen is being made
These markers are not yet common in skin trials, but they show where the field is heading: beyond “looks better” toward biological readouts.
The Takeaway: The closer research gets to these “hard” biological markers, the stronger the case becomes. Scepticism helps separate the “noise” from the meaningful data.
Reason 5 — Lifestyle context matters
Concede. Sceptics are right that no supplement, collagen included, is a magic wand. If your diet is poor, your sleep is broken, and you bake in the sun unprotected, a daily scoop won’t turn back time. A healthy diet and balanced diet are essential for supporting collagen production and maintaining healthy skin.
Counter. What research shows is that collagen works synergistically with its environment. Vitamin C is essential for collagen cross-linking; minerals like zinc and copper support the enzymes that stabilize connective tissue; adequate sleep and UV protection prevent breakdown; and balanced nutrition supplies the amino acids needed for repair. Clinical gains from supplementation are modest on their own but become more meaningful when they layer onto these foundations [Pullar et al., 2017]. You can read more about how to (Naturally Promote Collagen Production) here.
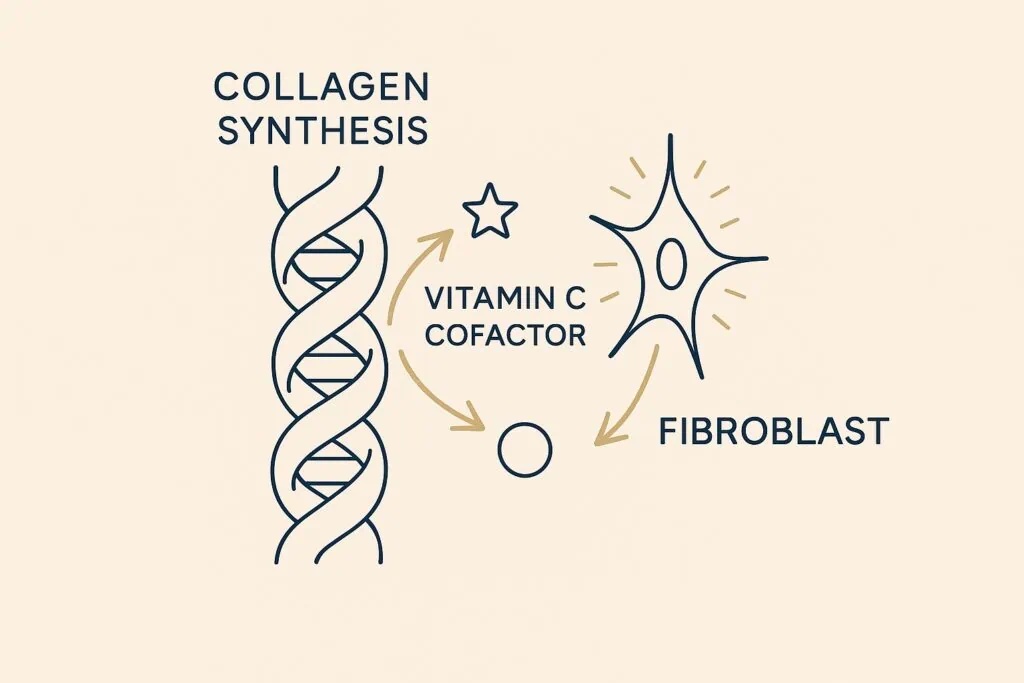
Collagen is best seen as an amplifier of renewal, not a standalone fix. Recent independent meta-analyses have helped clarify what collagen is—and what it is not. When evaluated purely on visible cosmetic endpoints, exaggerated claims do not hold up under unbiased scrutiny.
The research has recalibrated expectations, and rightfully so. It invites us to look beyond beauty marketing hype and focus on the validated mechanisms of collagen: biological signalling, peptide absorption, and long-term structural support. It challenges us to attune to our own biological blueprint.
Collagen is not a miracle. It is a conditional input, one that supports repair biology when the surrounding system allows it.
The most defensible claim for collagen peptides is not dramatic cosmetic change, but biological signaling. Peptides such as Pro-Hyp are absorbed intact, reach connective tissue, and interact with fibroblasts involved in repair and matrix maintenance. Whether this signaling translates into visible skin changes depends on the surrounding biological context; nutrition, inflammation, UV exposure, and time. Collagen does not override aging; it modestly biases the system toward repair when conditions allow.
Bridge — Where we stand
This article focuses on biological mechanisms, not guaranteed cosmetic miracles—because in complex systems like human skin, signalling does not equal certainty.
We share much of the sceptic’s lens. Funding bias exists. Not all powders are equal. We value transparency over hype, peptide-first thinking over vague promises, and context over magic bullets. That means clear dosing, third-party testing, and a focus on the mechanisms that have been independently shown to matter — especially low molecular weight peptides that deliver repair signals like Pro-Hyp.

Collagen is not a miracle. It is a tool — one that, when properly sourced, tested, and integrated, can play a real part in the biology of thriving.
FAQs
No, but many are. Independent labs — especially in Japan — have published mechanistic work showing Pro-Hyp and related peptides appear in blood after ingestion. Funding bias is real, which is why preregistration, objective instruments, and disclosure of conflicts are critical.
Collagen is a form of protein that you can get from food, specifically bone broths and slow-cooked meats. However, the collagen molecules in food are very large; native collagen is approximately 100- 300 kDa. Your body must break these long chains down extensively to attempt to absorb the resulting peptides.
Hydrolyzed collagen (HC) supplements provide collagen chains that have already been enzymatically broken down into small fragments, typically <5 kDa. This ensures significantly higher bioavailability; the proportion of the protein that is successfully absorbed into the bloodstream rather than just being passed through or fully broken down. This provides an immediate source of bioactive peptides, such as Pro-Hyp (Prolyl-Hydroxyproline). As shown by independent studies in Japan, these small peptides are absorbed intact via the PEPT1 and PEPT2 transporters. Furthermore, (HC) contains readily available hydroxyproline—a unique amino acid that is the primary biochemical marker of the collagen matrix and is rarely found in other protein sources.
Nutritionally, both supply the same building blocks. The difference is in source quality and transparency: bovine collagen in the EU is tightly regulated; marine collagen quality depends on contaminant testing and responsible sourcing. Safety comes down to documented supplier practices, not species alone. You can read more about (Marine vs Bovine collagen) in our dedicated guide here
Not intact. After supplementation, small peptides such as Pro-Hyp are detectable in human blood. These act as signals to fibroblasts, the cells that produce collagen in skin.
No. Collagen is not a magic fix. It works modestly on its own, but gains are more meaningful when combined with co-factors like vitamin C, sun protection, and healthy lifestyle habits. You can read more about how to (Naturally Promote Collagen Production) here.